A traditional italian appetizer is caprese, or tomato, mozzarella, and basil. There are many ways to make caprese, but the theme is generally the same. Why not have some fun with the dish?
The result of my experiment: Caprese with balsamic vinegar caviar.

What exactly is balsamic vinegar caviar, you might ask. Basically, balsamic vinegar in orbs that appear to the unknowing eye to look like caviar. The better question is, “What interesting chemistry is going on to create this?”
Vinegar is hydrophilic, meaning that it is made up of particles that are “water loving.” This is different from oil, which is hydrophobic, or “water hating.” Most people have seen this before when adding vinegar to olive oil. The two are immiscible, and will stay separate from each other. The lowest energy way for a droplet of vinegar in olive oil to do this is to form a sphere.

On a similar note, many people are familiar with the phrase “micelle”. It is the spherical shape that forms when a molecule has both hydrophilic and hydrophobic parts. It is based on the fact that chemically, “like favors like.” The hydrophilic parts will arrange themselves on the outside of the micelle where they can interact with the hydrophilic solvent (aka water). This will shield the hydrophobic parts, which can then have favorable interactions in the center of the sphere. This can be seen in molecules which have both “water-liking” and “water-hating” parts, such as in detergents or soap.
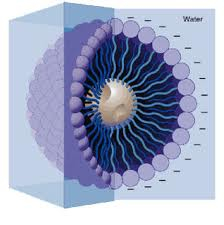
Using this knowledge, we can approximate what is happening on a chemical level when we make balsamic vinegar caviar.
Vinegar is made of mostly water and acetic acid.

Olive oil on the other hand is made up of triglyceride esters.
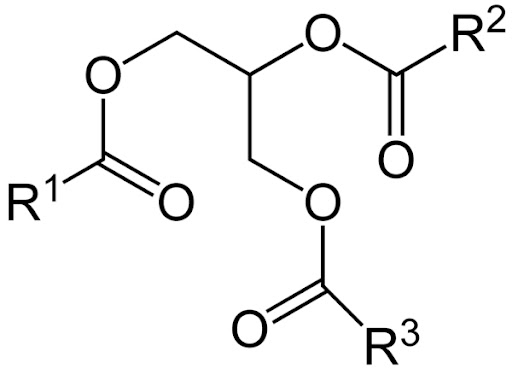
When vinegar is dropped into olive oil, it tends to stay together. It does this (not consciously but because it is lower energy) to decease the surface area that it shares with olive oil, and thereby lowering the amount of unfavorable interactions and increasing the favorable ones.
All of this is simple kitchen chemistry. But it gets more interesting when you throw in Agar. Agar, or Agar-agar as it is also known, is a combination of agarose and agaropectin.

Agar has very interesting chemical properties, which is why it has been used historically as a gelling agent. One of the cooler aspects of agar, is that it exhibits hysteresis. As defined by Wikipedia, hysteresis is, “The dependence of a system not only on its current environment but also on its past environment. This dependence arises because the system can be in more than one internal state. To predict its future development, either its internal state or its history must be known.” (Wikipedia)
What this means for agar is that once it is set as a gel, it takes a much higher temperature to convert the gel back to a liquid. Agar will melt at 185°F / 85°C. It will begin to solidify in the range of 90–104°F / 32–40°C. When agar melts, it is in a higher energy state. This allows it to relax into a single stranded molecule. When it cools down, these single stranded start form double helix structures. The ends of the double helix structures will bind together, creating a mesh matrix of agar. (Cooking for Geeks)
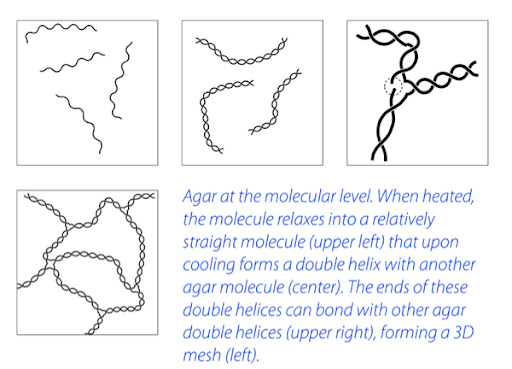
The agar allows for the spheres of vinegar that naturally form due to hydrophobic-hydrophilic interactions to solidify in this state. This creates a state of perpetual bliss, so to speak.
So, getting back to our balsamic vinegar caviar. To get prepared, a glass of olive oil should be put in the freezer for about 30 minutes. Then, the vinegar (around 100g/7 Oz) needs to be heated up to boiling. The agar (2 g) is added. The solution is mixed and allowed to cool down to 50-55 ˚C/120-130 ˚F. Once the vinegar solution is cooled down to about 50 ˚C, we’re ready to go.

Take the cold glass of olive oil out. Fill up a syringe with the vinegar-agar solution. Slowly drop droplets of the vinegar-agar into the olive oil. Droplets should form and solidify. Collect them with a slotted spoon from the bottom of the glass, wash in water, and enjoy!

Sources:
Wikipedia: Acetic Acid, Agar, Triglyceride Esters, Olive oil and Vinegar, Hysteresis
Molecular Recipes: http://www.molecularrecipes.com/gelification/balsamic-vinegar-pearls/
Micelle Image: http://dma980.com/phpSitemapNG/micelle-phospholipid
Cooking for Geeks: http://static.cookingforgeeks.com/press/press-kits/cooking-for-geeks-p310-311-agar.pdf
Leave a Reply